|
MIKROLATEST®
BMD Single-Strip kits High quality system for antibiotic susceptibility testing based on minimum inhibitory concentration determination adjusted to visual or automated reading. Each kit consists of 3 x 96 well plates containing 12 x 8 well removable strips. Each strip containing 7 dilutions of a specific antibiotic and 1 well for purposes of a growth control. Currently the range consists of 20 antibiotics in single strip format. Both Single-Strips and predefined panels are available. |
The Erba Mikrolatest accessories improve the accuracy and reproducibility of single-strip broth microdilution (SS-BMD).
ErbaScan reader removes subjective by-eye reading and records results. ErbaExpert software EUCAST and CLSI interprets and record with yearly updates. Densilameter helps standardise inoculum. ErbaFill offers automated filling of the strips and 96 well plates. |
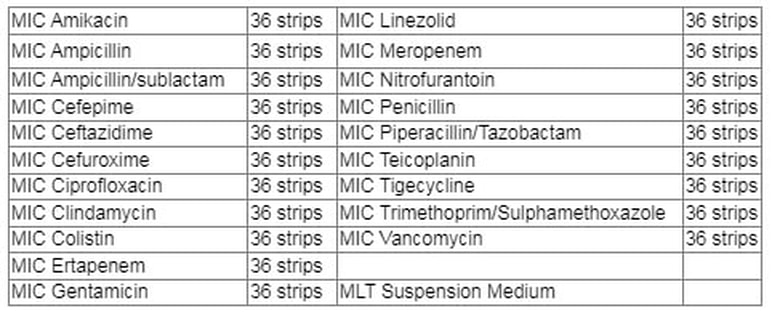
Current single-strip Broth Microdilution tests available from the MIKROLATEST range
BIOCONNECTIONS introduced Single-Strip Broth Microdilution (SS-BMD) products in 2014 starting with Colistin in response to EUCAST warnings of other methods. This was followed by Vancomycin/Teicoplanin and Piperacillin Tazobactam. There are now over 60 UK laboratories using SS-BMD products. We can now offer an extended range of 20 SS-BMD products together with accessories.
Erba Lachema, producers of Mikrolatest, are a subsidiary of Erba Mannhein a large multinational life sciences company. Based in the Czech Republic they have been manufacturing BMD products for several years. As well as the 20 SS-BMD strips they also have a range of 96 well plate format BMD for Gram negative (including non-fermenters) and Gram positive isolates.
The quality of Erba Mikrolatest is well established. In addition to the acceptance of the product in UK laboratories, a member of the EUCAST general committee confirmed the performance of the Colistin SS-BMD. All the products in the Erba Mikrolatest range carry current CE-IVD certification/registration and are registered for use with MHRA. These products were launched in the UK in March and several UK laboratories are already using the Erba Mikrolatest SS-BMD products. Erba Lachema, producers of Mikrolatest, are a subsidiary of Erba Mannhein a large multinational life sciences company.
As well as the 20 SS-BMD strips the MIKROLATEST range includes 96 well plate format BMD plates. The following is an outline. More information is available on the Erba Lachema website.
https://bit.ly/3GNSsKG
Erba Lachema, producers of Mikrolatest, are a subsidiary of Erba Mannhein a large multinational life sciences company. Based in the Czech Republic they have been manufacturing BMD products for several years. As well as the 20 SS-BMD strips they also have a range of 96 well plate format BMD for Gram negative (including non-fermenters) and Gram positive isolates.
The quality of Erba Mikrolatest is well established. In addition to the acceptance of the product in UK laboratories, a member of the EUCAST general committee confirmed the performance of the Colistin SS-BMD. All the products in the Erba Mikrolatest range carry current CE-IVD certification/registration and are registered for use with MHRA. These products were launched in the UK in March and several UK laboratories are already using the Erba Mikrolatest SS-BMD products. Erba Lachema, producers of Mikrolatest, are a subsidiary of Erba Mannhein a large multinational life sciences company.
As well as the 20 SS-BMD strips the MIKROLATEST range includes 96 well plate format BMD plates. The following is an outline. More information is available on the Erba Lachema website.
https://bit.ly/3GNSsKG
MIC G-I
The kit is designed to test antimicrobial susceptibility of bacteria from Enterobacteriaceae family.
MIC G-II
This kit is designed to test susceptibility to antibiotics used in treatment of serious infections caused by bacteria from Enterobacteriaceae family especially in hospitalized patients.
MIC URINE
The kit is designed to test antimicrobial susceptibility of bacteria isolated from urine and urinary tract (mainly Enterobacteriaceae family).
MIC NEFERM
The kit is designed to test antimicrobial susceptibility of non-fermenting bacteria.
MIC STAPHY
The kit is designed to test antimicrobial susceptibility of staphylococci.
MIC G+
The kit is designed to test antimicrobial susceptibility of Gram-positive bacteria: streptococci A,B,C, G, Streptococcus pneumoniae and enterococci.
The kit is designed to test antimicrobial susceptibility of bacteria from Enterobacteriaceae family.
MIC G-II
This kit is designed to test susceptibility to antibiotics used in treatment of serious infections caused by bacteria from Enterobacteriaceae family especially in hospitalized patients.
MIC URINE
The kit is designed to test antimicrobial susceptibility of bacteria isolated from urine and urinary tract (mainly Enterobacteriaceae family).
MIC NEFERM
The kit is designed to test antimicrobial susceptibility of non-fermenting bacteria.
MIC STAPHY
The kit is designed to test antimicrobial susceptibility of staphylococci.
MIC G+
The kit is designed to test antimicrobial susceptibility of Gram-positive bacteria: streptococci A,B,C, G, Streptococcus pneumoniae and enterococci.
|
|
|