SARS-CoV-2
IVD Quality Controls
COVID-19 has been a disruptive force worldwide. Diagnostic manufacturers and clinical
laboratories have responded to the shear enormity of the pandemic with a proliferation of
tests. For these molecular methodologies, providing an unbiased assessment of assay performance is critical. Microbiologics portfolio of SARS-CoV-2 quality controls provides laboratories with the confidence needed for their SARS-CoV-2 test results.
**NEW - SARS-CoV-2 Process Controls and Inactivated Whole Virus Controls**
Microbiologics launched the first IVD molecular control for
SARS-CoV-2. in March 2020.
SARS-CoV-2. in March 2020.
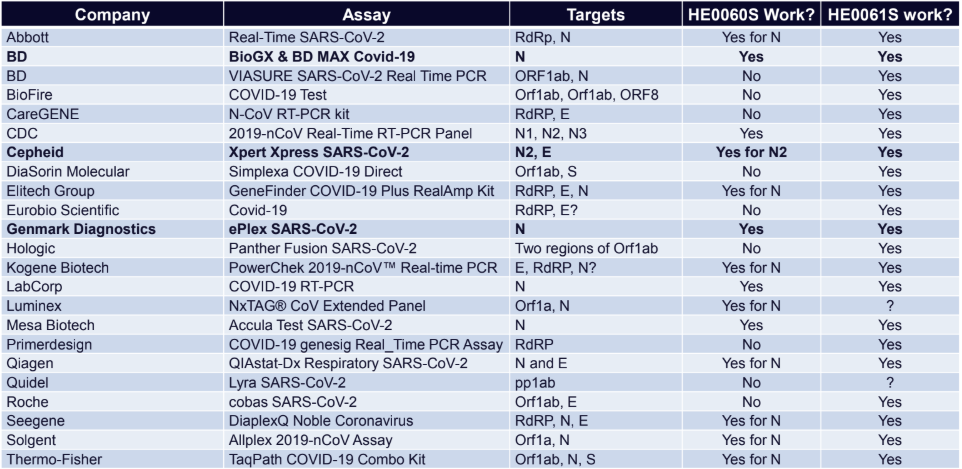
More Targets Covered means more Systems Covered
Compatability Chart
Format
Dried RNA Preserved with Biomatrica RNAstable®
IVD Helix Elite™
Synthetic Standard Format. Biosafety level 1
No health and safety risk from exposure
Catalog Number HE0060S
CE marked IVD registered
Dried RNA Preserved with Biomatrica RNAstable®
IVD Helix Elite™
Synthetic Standard Format. Biosafety level 1
No health and safety risk from exposure
Catalog Number HE0060S
CE marked IVD registered
About Helix Elite™
Molecular diagnostic tests offer rapid and specific information regarding the presence and quantity of a microorganism (e.g., bacterium, parasite, virus, etc).
Development and proper interpretation of a molecular diagnostic test requires the use of a positive control. A positive control confirms the proper performance of a molecular assay and operator.
Synthetic Helix Elite™ Molecular Standards are nucleic acids created for use as positive control surrogates for various microorganisms and viruses where target genomic material may be difficult or unsafe to obtain.
Molecular diagnostic tests offer rapid and specific information regarding the presence and quantity of a microorganism (e.g., bacterium, parasite, virus, etc).
Development and proper interpretation of a molecular diagnostic test requires the use of a positive control. A positive control confirms the proper performance of a molecular assay and operator.
Synthetic Helix Elite™ Molecular Standards are nucleic acids created for use as positive control surrogates for various microorganisms and viruses where target genomic material may be difficult or unsafe to obtain.
|
|
|