Candida auris is on the WHO fungal priority pathogens list; where the 4 'critical threat' pathogens are those ranked highest for perceived public health importance (Cryptococcus neoformans, Candida auris, Aspergillus fumigatus and Candida albicans)
|
Key Characteristics of eazyplex® Candida auris Direct diagnosis from swabs or culture confirmation in just 25 minutes. TATs under 30 minutes Accuracy Ease of use, No DNA extraction Suitable for single or small numbers of tests Samples from rectal swab (specified), and agar plate. Published evaluation
Simple differentiation of Salmonella Typhi, Paratyphi and Choleraesuis from Salmonella species using the eazyplex TyphiTyper LAMP assay PMID: 32459614 Koch Institute Germany 2020 Conclusion. The eazyplex TyphiTyper is very easy to perform and allows the rapid identification of TS and S. Choleraesuis isolates. |
eazyplex®Candida albicans
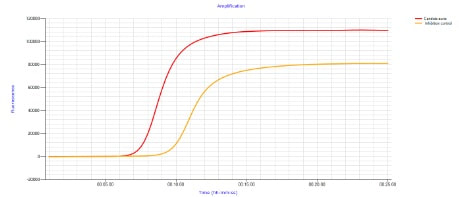
he graph of the detection of a Candida auris is shown. The detection directly from the sample (swab) is reported here as positive after 7:35 minutes. The eazyplex test system is a freeze dried ready to use amplification reaction and can be stored direct at the workplace. During the amplification the result is shown in Real-Time.
Within 25 min you have all the details! |

The Genie Platforms for eazyplex®
Genie® II and the Genie® HT are the current models.
The compact Genie II is a light and robust device for rapid individual tests 24/7. The device has two heating blocks. The blocks can be controlled independntly of each other and thus offer maximum flexibility.
The Genie® HT is a modular upgrade with the option of 1 up to 12 independant working blocks for high throghput settings.
Two processing devices are currently available with interesting developments following.
The ingenious Genie® allows you to carry out fast and highly sensitive molecular diagnostics of DNA and RNA.
This powerful and extremely flexible platform enables isothermal amplification of all DNA and RNA targets in one compact device. It was specially developed for isothermal amplification assays with real-time detection based on fluorescence.
Genie® II and the Genie® HT are the current models.
The compact Genie II is a light and robust device for rapid individual tests 24/7. The device has two heating blocks. The blocks can be controlled independntly of each other and thus offer maximum flexibility.
The Genie® HT is a modular upgrade with the option of 1 up to 12 independant working blocks for high throghput settings.
Two processing devices are currently available with interesting developments following.
The ingenious Genie® allows you to carry out fast and highly sensitive molecular diagnostics of DNA and RNA.
This powerful and extremely flexible platform enables isothermal amplification of all DNA and RNA targets in one compact device. It was specially developed for isothermal amplification assays with real-time detection based on fluorescence.
The Amplex Genie & eazyplex® open a door to a wide range of LAMP diagnostics, offering TATs under 30 minutes.
Introducing Amplex eazyplex® LAMP assays to your hospital opens the door to a wide range of molecular diagnostics.
For example in under 30 minutes the eazyplex®Blood Screen will provide first steps in your ?septicaemia case. The range of assays include others that all require urgent action such as meningitis, STIs, ARMs, Candida auris etc.
Introducing Amplex eazyplex® LAMP assays to your hospital opens the door to a wide range of molecular diagnostics.
For example in under 30 minutes the eazyplex®Blood Screen will provide first steps in your ?septicaemia case. The range of assays include others that all require urgent action such as meningitis, STIs, ARMs, Candida auris etc.
The manufacturer
Amplex Diagnostics GmbH is a German-based company founded in 2002 that specialises in providing rapid and robust CE-IVD tests to aid the diagnosis of microbiological infections. One of their products was the world's first MRSA multiplex PCR test to be CE IVD-certified for testing directly from sample material.
Amplex makes the latest advanced technology available in a simple and user-friendly manner. Their eazyplex® system which can be operated intuitively, combines isothermal amplification (LAMP) with real-time detection. The eazyplex® reagents are supported by the Genie platforms which enables isothermal amplification of all DNS and RNA targets in one compact device. It was specially developed for isothermal amplification assays with real-time detection based on fluorescence and enables reading of molecular diagnostics Real-time detection within a few minutes.
www.eazyplex.com
Amplex Diagnostics GmbH is a German-based company founded in 2002 that specialises in providing rapid and robust CE-IVD tests to aid the diagnosis of microbiological infections. One of their products was the world's first MRSA multiplex PCR test to be CE IVD-certified for testing directly from sample material.
Amplex makes the latest advanced technology available in a simple and user-friendly manner. Their eazyplex® system which can be operated intuitively, combines isothermal amplification (LAMP) with real-time detection. The eazyplex® reagents are supported by the Genie platforms which enables isothermal amplification of all DNS and RNA targets in one compact device. It was specially developed for isothermal amplification assays with real-time detection based on fluorescence and enables reading of molecular diagnostics Real-time detection within a few minutes.
www.eazyplex.com
|
|
|