WHO has declared that AMR is one of the top 10 global public health threats facing humanity. Misuse and overuse of antimicrobials are the main drivers in the development of drug resistant pathogens. TIME FOR CHANGE
The eazyplex® SuperBug series is a direct response to these challenges! The eazyplex® tests are easy-to-use and user-friendly molecular tests that detects resistance targets under 30 minutes directly from sample material.
These rapid diagnostic tests cover both prevalent Gram negative resistances (such as KPC, NDM, and CTX-M) and the less prevalent yet equally important resistance genes like GIM, GES, and AmpC. So simple to use they can be considered almost POCT.
The eazyplex® SuperBug series is a direct response to these challenges! The eazyplex® tests are easy-to-use and user-friendly molecular tests that detects resistance targets under 30 minutes directly from sample material.
These rapid diagnostic tests cover both prevalent Gram negative resistances (such as KPC, NDM, and CTX-M) and the less prevalent yet equally important resistance genes like GIM, GES, and AmpC. So simple to use they can be considered almost POCT.
|
The eazyplex® SuperBug series
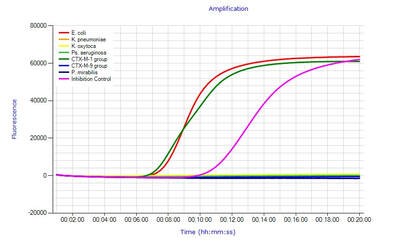
The eazyplex® SuperBug series is a molecular identification product based on LAMP technology. Key characteristics are; TATs under 30 minutes Ease of use, No DNA extraction Suitable for single or small numbers of tests Samples from urine, rectal swab (specified), blood culture (specified) and agar plate. Accuracy This column illustrates the development of eazyplex® over the last decade. There are many more published evaluations on the Amplex website These published evaluations (and more) of eazyplex® can be accessed at eazyplex® website-publications. |
This column illustrates the development of eazyplex® over the last decade.
These evaluations, and many more evaluations, of eazyplex®SuperBug series can be accessed at eazyplex® website-publications. 2015 Evaluation of three commercial assays for rapid detection of genes encoding clinically relevant carbapenemases in cultured bacteria. This publication came from Public Health England in 2015. doi:10.1093/jac/dku571 ''Commercial assays offer a reliable means of detecting bacteria with clinically significant carbapenemases. Coverage of some assays required expansion to maximize the sensitivity for OXA-48-like carbapenemases. Choice will ultimately depend on preferred gene coverage, intended throughput, cost and ability to fit into local workflows.'' 2015 Extended-spectrum β-lactamase (ESBL) detection directly from urine samples with the rapid isothermal amplification-based eazyplex® SuperBug CRE assay: Proof of concept.
from University Hospital Basel, 2015 https://pubmed.ncbi.nlm.nih.gov/26506282/ ''A commercially available assay (eazyplex® SuperBug CRE) detecting the most common carbapenemase and ESBL types was evaluated directly on 50 urine samples. Eazyplex® correctly detected ESBL-encoding genes in all 30 urine samples with confirmed ESBL production (sensitivity 100%). Two specimens showed invalid and one specimen false-positive results (specificity 97.9%).'' 2022 The Evaluation of Eazyplex® SuperBug CRE Assay Usefulness for the Detection of ESBLs and Carbapenemases Genes Directly from Urine Samples and Positive Blood Cultures
from Nicolaus Copernicus University, Poland. https://doi.org/10.3390/antibiotics11020138 ''The Eazyplex® SuperBug CRE assay is based on a loop-mediated isothermal amplification of genetic material and allows for the detection of a selection of genes encoding carbapenemases, KPC, NDM, VIM, OXA-48, OXA-181 and extended-spectrum beta-lactamases from the CTX-M-1 and CTX-M-9 groups. A total of 120 clinical specimens were included in the study. The test gave valid results for 58 (96.7%) urine samples and 57 (95.0%) positive blood cultures. ESBL and/or carbapenemase enzymes genes were detected in 56 (93.3%) urine and 55 (91.7%) blood samples, respectively. The Eazyplex® SuperBug CRE assay can be used for a rapid detection of the genes encoding the most important resistance mechanisms to beta-lactams in Gram-negative rods also without the necessity of bacterial culture'' 2023 Screening of Klebsiella pneumoniae Isolates for Carbapenemase and Hypervirulence-Associated Genes by Combining the Eazyplex® Superbug CRE and hvKp Assays
from Jena University Hospital, Robert Koch Institute, and Sophien Klinikum, Germany https://doi.org/10.3390/antibiotics12060959 ''The time for the results of the eazyplex® assays ranged from 6.5 to 13 min, and the total turnaround time, including sample preparation, was less than 30 min.'' hypervirulent K. pneumoniae (hvKp) - Diagnostics should not be restricted to carbapenem-resistant isolates. Non-carbapenem-resistant hvKp can also cause severe invasive infections, and carbapenemase-producing K. pneumoniae can acquire hypervirulence-associated genes by plasmid transfer. The eazyplex® test results for beta-lactamase and virulence genes were confirmed. The eazyplex® hvKp, currently only available as a Research Use Only assay, may be a useful tool for the rapid identification of hvKp without significant additional workload when combined with the eazyplex® Superbug CRE assay for the detection of carbapenemases and for Acinetobacter
2021 Evaluation of the Amplex eazyplex® SuperBug Acineto test for detection of acquired OXA and NDM carbapenemases in Acinetobacter spp from Detection of Antimicrobial Resistance Unit, University Hospital of North Norway, Tromsø. DOI:10.1016/j.jhin.2021.09.015 ''In conclusion, the results show that the eazyplex® SuperBug Acineto test is a rapid (15–30 min) and reliable test for the detection of the most common carbapenemases observed in Acinetobacter spp. and for species determination of A. baumannii.. The eazyplex® SuperBug Acineto test correctly identified all carbapenemase genes, including in strains co-harbouring two targeted acquired carbapenemase genes compared on par with the WGS results. No false-positive reactions were observed.'' 2021 Endemicity of OXA-23 and OXA-72 in clinical isolates of Acinetobacter baumannii from three neighbouring countries in Southeast Europe. various Institutes in Croatia, Herzegovina and Serbia. PMID: 33502723 |
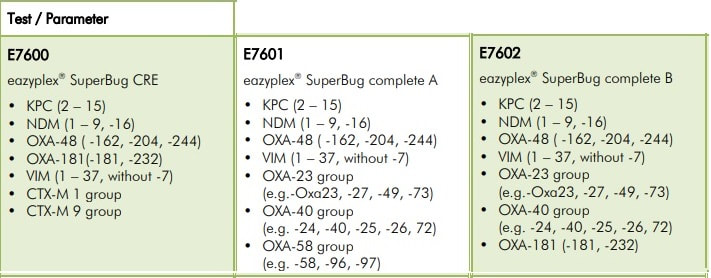
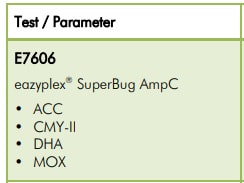
This is the current eazyplex® SuperBug product line
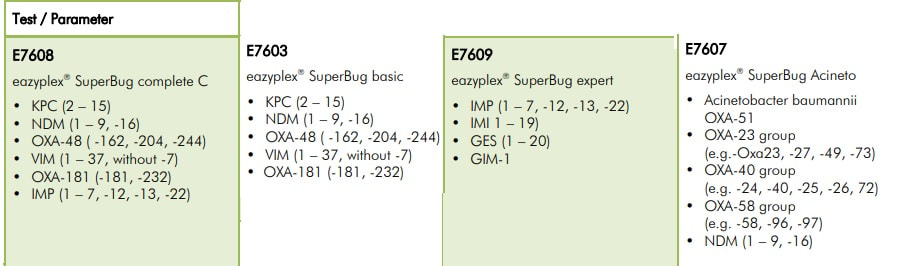
Many parameters are available to you in the eazyplex® SuperBug series.
Hands-on-time approx. 3 min.
All signals are displayed to you in real time during the amplification.
The eazyplex® test system is a lyophilized, ready-to-use amplification system and can be stored directly at the workplace at room temperature.
Many parameters are available to you in the eazyplex® SuperBug series.
Hands-on-time approx. 3 min.
- First signals in less than 10 min.
- Complete test run finished in 20 min.
- Results output as PDF, CSV and adhesive label
All signals are displayed to you in real time during the amplification.
The eazyplex® test system is a lyophilized, ready-to-use amplification system and can be stored directly at the workplace at room temperature.
The eazyplex® panels line offers much more
Introducing Amplex eazyplex® LAMP assays to your hospital opens the door to a wide range of molecular diagnostics.
For example in under 30 minutes the eazyplex®Blood Screen will provide first steps in your ?septicaemia case. The range of assays include others that all require urgent action such as meningitis, STIs, ARMs, Candida auris etc.
Introducing Amplex eazyplex® LAMP assays to your hospital opens the door to a wide range of molecular diagnostics.
For example in under 30 minutes the eazyplex®Blood Screen will provide first steps in your ?septicaemia case. The range of assays include others that all require urgent action such as meningitis, STIs, ARMs, Candida auris etc.
eazyplex® panels publications
The eazyplex® panels publications library includes over 25 publications from 9 European countries.
The eazyplex® panels publications library includes over 25 publications from 9 European countries.
To access the eazyplex® panels publications library - click on the graphic above.
The products covered are relevant to acute infectious diseases such as septicaemia, pneumonia, and meningitis, also covered are specific key pathogens such as C. auris, MRSA, C. difficile, Salmonella typhi, SARs-CoV-2, Pneumocystis jirovecii and EHEC.
Resistance to antibiotics in pathogens is widely covered, particularly in Gram-negatives. The eazyplex® SuperBug series not only cover the common resistances such as KPC, NDM, AmpC and CTX-M, but cover resistance genes, such as GIM and GES which are still rare at the moment. The Blood Screen panel not only detects the most common bacterial pathogens of sepsis but also associated CTX-M-1 and CTX -M-9 genes.
Acinetobacter: Multidrug-resistant Acinetobacter baumannii is considered a major public-health threat owing to limited treatment options. Results of a published evaluation show that the eazyplex® SuperBug Acineto test is a rapid (15–30 min) and reliable test for the detection of the most common carbapenemases observed in Acinetobacter spp. and for species determination of A. baumannii.
The products covered are relevant to acute infectious diseases such as septicaemia, pneumonia, and meningitis, also covered are specific key pathogens such as C. auris, MRSA, C. difficile, Salmonella typhi, SARs-CoV-2, Pneumocystis jirovecii and EHEC.
Resistance to antibiotics in pathogens is widely covered, particularly in Gram-negatives. The eazyplex® SuperBug series not only cover the common resistances such as KPC, NDM, AmpC and CTX-M, but cover resistance genes, such as GIM and GES which are still rare at the moment. The Blood Screen panel not only detects the most common bacterial pathogens of sepsis but also associated CTX-M-1 and CTX -M-9 genes.
Acinetobacter: Multidrug-resistant Acinetobacter baumannii is considered a major public-health threat owing to limited treatment options. Results of a published evaluation show that the eazyplex® SuperBug Acineto test is a rapid (15–30 min) and reliable test for the detection of the most common carbapenemases observed in Acinetobacter spp. and for species determination of A. baumannii.

The Genie Platforms
Two processing devices are currently available with interesting developments following.
The ingenious Genie® allows you to carry out fast and highly sensitive molecular diagnostics of DNA and RNA.
This powerful and extremely flexible platform enables isothermal amplification of all DNS and RNA targets in one compact device. It was specially developed for isothermal amplification assays with real-time detection based on fluorescence.
Genie® II and the Genie® HT are the current models.
The compact Genie II is a light and robust device for rapid individual tests 24/7. The device has two heating blocks. The blocks can be controlled independntly of each other and thus offer maximum flexibility.
The Genie® HT is a modular upgrade with the option of 1 up to 12 independant working blocks for high throghput settings.
Two processing devices are currently available with interesting developments following.
The ingenious Genie® allows you to carry out fast and highly sensitive molecular diagnostics of DNA and RNA.
This powerful and extremely flexible platform enables isothermal amplification of all DNS and RNA targets in one compact device. It was specially developed for isothermal amplification assays with real-time detection based on fluorescence.
Genie® II and the Genie® HT are the current models.
The compact Genie II is a light and robust device for rapid individual tests 24/7. The device has two heating blocks. The blocks can be controlled independntly of each other and thus offer maximum flexibility.
The Genie® HT is a modular upgrade with the option of 1 up to 12 independant working blocks for high throghput settings.

The manufacturer
Amplex Diagnostics GmbH is a German-based company founded in 2002 that specialises in providing rapid and robust CE-IVD tests to aid the diagnosis of microbiological infections. One of their products was the world's first MRSA multiplex PCR test to be CE IVD-certified for testing directly from sample material.
Amplex makes the latest advanced technology available in a simple and user-friendly manner. Their eazyplex® system which can be operated intuitively, combines isothermal amplification (LAMP) with real-time detection. The eazyplex® reagents are supported by the Genie platforms which enables isothermal amplification of all DNS and RNA targets in one compact device. It was specially developed for isothermal amplification assays with real-time detection based on fluorescence and enables reading of molecular diagnostics Real-time detection within a few minutes.
www.eazyplex.com
Amplex Diagnostics GmbH is a German-based company founded in 2002 that specialises in providing rapid and robust CE-IVD tests to aid the diagnosis of microbiological infections. One of their products was the world's first MRSA multiplex PCR test to be CE IVD-certified for testing directly from sample material.
Amplex makes the latest advanced technology available in a simple and user-friendly manner. Their eazyplex® system which can be operated intuitively, combines isothermal amplification (LAMP) with real-time detection. The eazyplex® reagents are supported by the Genie platforms which enables isothermal amplification of all DNS and RNA targets in one compact device. It was specially developed for isothermal amplification assays with real-time detection based on fluorescence and enables reading of molecular diagnostics Real-time detection within a few minutes.
www.eazyplex.com